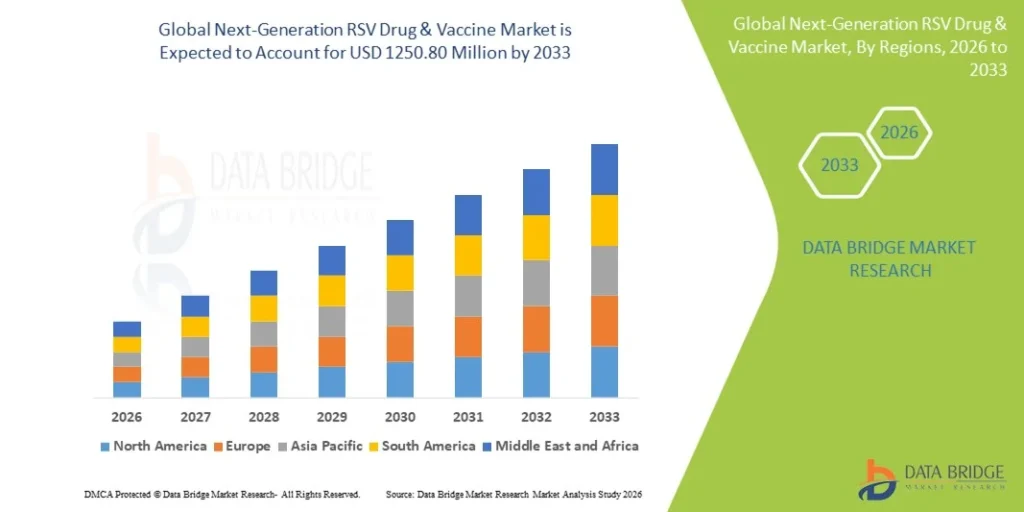
The Global Next-Generation RSV Drug and Vaccine Market was valued at USD 594.00 Million in 2025 and is expected to reach USD 1,250.80 Million by 2033, growing at a CAGR of 9.8% (2026-2033).
Introduction
Respiratory Syncytial Virus (RSV) has long been one of the most common and underestimated – respiratory viruses worldwide. Nearly every child is infected by RSV before the age of two, and while many cases resemble a mild cold, RSV can cause severe lower respiratory tract disease, including bronchiolitis and pneumonia. Infants, older adults, pregnant women, and people with compromised immune systems are particularly vulnerable. For decades, prevention and treatment options were extremely limited. Today, however, a new generation of RSV drugs and vaccines is reshaping how we prevent, manage, and think about this virus.
Definition
A Next-Generation RSV Drug and Vaccine refers to advanced therapeutic and preventive solutions designed to more effectively combat respiratory syncytial virus (RSV) by leveraging modern technologies such as enhanced monoclonal antibodies, novel antiviral agents, and innovative vaccine platforms. These next-generation approaches aim to provide broader and longer-lasting protection, improved safety and efficacy, and expanded coverage across vulnerable populations, including infants, older adults, and immunocompromised individuals, compared with earlier RSV treatments and vaccines.
A Brief Look at RSV and Past Limitations
RSV is a highly contagious virus spread through respiratory droplets and direct contact. Seasonal outbreaks typically occur during fall and winter, placing heavy strain on pediatric wards and intensive care units. Despite its impact, RSV research lagged behind other viral diseases such as influenza or COVID-19.
For many years, the only preventive option was palivizumab, a monoclonal antibody given monthly during RSV season. While effective, it was expensive, required repeated injections, and was limited to high-risk infants. There were no approved vaccines, and treatment options focused mainly on supportive care such as oxygen and hydration.
Several early vaccine attempts failed due to safety issues, including a notorious 1960s trial in which a poorly designed RSV vaccine led to more severe illness in vaccinated children. This history made researchers cautious and slowed progress for decades.
Why RSV Research Has Accelerated Now
Multiple factors have contributed to the recent surge in RSV innovation:
- Advances in structural biology that clarified how RSV enters human cells
- Improved vaccine platforms, including mRNA and protein subunit technologies
- Lessons learned from COVID-19, which accelerated clinical trial design and regulatory pathways
- Growing awareness of RSV burden, particularly in older adults
These developments have led to safer, more targeted approaches that are now reaching the market.
Next-Generation RSV Vaccines: What’s New?
1. Targeting the Prefusion F Protein
One of the most important scientific breakthroughs in RSV research was the discovery of the prefusion conformation of the RSV F (fusion) protein. This protein helps the virus fuse with human cells. Earlier vaccines targeted the postfusion form, which did not generate strong protective immunity.
Next-generation vaccines stabilize the F protein in its prefusion shape, triggering a much stronger immune response. This innovation dramatically improved vaccine effectiveness and safety.
-
RSV Vaccines for Older Adults
For the first time, RSV vaccines are now approved for adults aged 60 and older. These vaccines significantly reduce the risk of severe RSV-related lower respiratory tract disease, hospitalizations, and complications such as pneumonia.
Older adults often experience RSV as seriously as influenza or COVID-19, but RSV historically went undiagnosed. Vaccination fills a major gap in adult respiratory care and reduces strain on healthcare systems during peak seasons.
-
Maternal RSV Vaccination
Another major advancement is vaccination during pregnancy. Maternal RSV vaccines are designed to protect newborns during their first months of life – when they are most vulnerable – by transferring antibodies through the placenta.
This approach mirrors successful strategies used for influenza and whooping cough. Instead of vaccinating infants directly, maternal immunization provides immediate passive immunity, reducing severe illness and hospitalization rates in early infancy.
-
Diverse Vaccine Platforms
Unlike older approaches, next-generation RSV vaccines use a variety of platforms:
- Protein subunit vaccines (highly targeted and well tolerated)
- mRNA-based vaccines (fast to adapt and highly immunogenic)
- Vector-based vaccines (leveraging viral carriers for immune stimulation)
This diversity allows for tailored vaccines across age groups and risk profiles.
Next-Generation RSV Drugs and Antibody Therapies
Vaccines are not the only innovation transforming RSV prevention. New long-acting monoclonal antibodies represent a major leap forward, particularly for infants.
1. Long-Acting Monoclonal Antibodies
Unlike palivizumab, which required monthly injections, newer monoclonal antibodies provide protection with a single dose per RSV season. These antibodies are engineered to last longer in the bloodstream and neutralize the virus more efficiently.
They are designed for broad infant populations, not just those at high risk. This could significantly reduce RSV-related hospitalizations in the first year of life.
-
Antiviral Drug Development
Researchers are also exploring direct-acting antiviral drugs that inhibit RSV replication. While still in earlier stages compared to vaccines and antibodies, these treatments may eventually offer therapeutic options for severe RSV infections, especially in immunocompromised patients.
Such drugs could reduce disease severity, shorten hospital stays, and improve outcomes when administered early.
How These Advances Change RSV Prevention Strategies
The arrival of next-generation RSV drugs and vaccines marks a shift from reactive to proactive care.
A Life-Course Approach:
RSV prevention is no longer limited to a narrow population. Strategies now span the entire life course:
- Pregnant individuals protect newborns
- Infants receive long-acting antibodies
- Older adults receive seasonal vaccination
- High-risk populations benefit from layered protection
This integrated approach mirrors modern strategies used for influenza and pneumococcal disease.
Reduced Healthcare Burden:
RSV hospitalizations account for a significant portion of winter healthcare demand, especially in pediatric units. Broad prevention can reduce:
- Emergency room visits
- Hospital admissions
- ICU utilization
- Healthcare costs
This is particularly important as health systems face staffing shortages and seasonal surges.
Improved Awareness and Diagnosis:
With vaccines and preventive therapies available, RSV is receiving more attention from clinicians and the public. Increased testing and diagnosis help ensure timely care and better surveillance, leading to more accurate public health planning.
Safety and Ongoing Monitoring
Modern RSV vaccines and antibodies have undergone large-scale clinical trials with rigorous safety monitoring. Regulatory agencies continue to track real-world effectiveness and rare side effects, especially as these products are used in broader populations.
Ongoing research is also focused on:
- Duration of protection
- Need for boosters
- Effectiveness against emerging RSV strains
- Co-administration with other vaccines
This continuous evaluation ensures that RSV prevention strategies remain effective and safe.
Why This Matters Now and in the Future
RSV is not a new virus, but our ability to fight it effectively is new. These next-generation drugs and vaccines represent more than scientific success—they reflect a shift in how we prioritize respiratory health across age groups.
Key reasons this progress matters include:
- Protecting the most vulnerable, especially infants and older adults
- Reducing preventable hospitalizations and deaths
- Strengthening healthcare resilience during respiratory virus seasons
- Setting a precedent for tackling other neglected viral diseases
As RSV prevention becomes more widespread, its overall burden is expected to decline significantly, much like what occurred with other vaccine-preventable diseases.
Growth Rate of Next-Generation RSV Drug and Vaccine Market
According to Data Bridge Market Research, the next-generation RSV Drug & vaccine market was estimated to be worth USD 594 million in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 9.80% to reach USD 1250.80 million by 2033.
Learn More: https://www.databridgemarketresearch.com/reports/global-next-generation-rsv-drug-and-vaccine-market
Conclusion
The emergence of next-generation RSV drugs and vaccines marks a turning point in the fight against a virus that has affected millions for decades. By leveraging advances in molecular biology, immunology, and vaccine technology, researchers have transformed RSV prevention from a limited, high-risk intervention into a comprehensive, life-course strategy.




